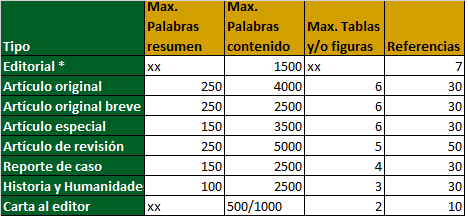
Anafilaxia a opioides en un paciente oncológico pediátrico: reporte de caso
DOI:
https://doi.org/10.37711/rpcs.2020.2.3.197Palabras clave:
humanos, tramadol, analgésicos, opioides síndrome de DownResumen
Se presenta el caso de un paciente de 5 años con diagnóstico de leucemia linfoblástica aguda y trisomía 21, quien fue hospitalizado para manejo con quimioterapia de primera línea en quien se presentaron diversas complicaciones durante su tratamiento, por lo que requirió manejo antibiótico y analgésico opioide. Posteriormente, desarrolla cuatro reacciones alérgicas; tres de ellas asociadas a opioides y una catalogada como angioedema severo relacionado con tramadol. Se presenta el caso clínico debido a la baja incidencia de reacciones severas alérgicas a opioides, particularmente al tramadol en el contexto de pacientes de alta complejidad con polimedicación.
Descargas
Referencias
1. Patanwala AE, Keim SM, Erstad BL. Intravenous opioids for severe acute pain in the emergency department. The Annals of pharmacotherapy. 2010; 44(11): 1800-1809. https://doi.org/10.1345/aph.1P438
2. Meng J., Rotiroti G, Burdett E, Lukawska JJ. Anaphylaxis during general anaesthesia: experience from a drug allergy centre in the UK. Acta anaesthesiologica Scandinavica. 2017; 61(3): 281–289. https://doi.org/10.1111/aas.12858
3. Ariosto D. Factors Contributing to CPOE Opiate Allergy Alert Overrides. AMIA Annu Symp Proc [Internet] 2014; 2014: 256-265. Disponible en: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4419937/
4. Kartal M, Oktay C, Bacanli A, karadeniz O. A Rare Diagnosis in Emergency Department: Morphine Related Anaphylactoid Reaction. Turkish Journal of Emergency Medicine [Internet] 2009; 9(4): 181-183. Disponible en: https://www.turkjemergmed.com/abstract/331
5. Montañez MI, Mayorga C, Bogas G, Barrionuevo E, Fernandez-Santamaria R, Martin-Serrano A, et al. Epidemiology, Mechanisms, and Diagnosis of Drug-Induced Anaphylaxis. Front. Immunol. 2017 May 29; 8:614. doi: 10.3389/fimmu.2017.00614
6. Baldo BA, Pham NH. Histamine-releasing and allergenic properties of opioid analgesic drugs: resolving the two. Anaesth Intensive Care. 2012; 40(2): 216-235. https://doi.org/10.1177/0310057X1204000204
7. Zhang B, Li Q, Shi C, Zhang X. Drug-Induced Pseudoallergy: A Review of the Causes and Mechanisms. Pharmacology. 2018; 101(1-2): 104–110. https://doi.org/10.1159/000479878
8. Gilbar PJ, Ridge AM. History of opioid allergy: what significance? J Oncol Pharm Pract. 2004; 10(3): 183-6. https://doi.org/10.1191/1078155204jp131oa
9. Kemp SF, Lockey RF. Anaphylaxis: a review of causes and mechanisms. The Journal of allergy and clinical immunology. 2002; 110(3): 341–348. https://doi.org/10.1067/mai.2002.126811
10. Mori F, Barni S, Manfredi M, Sarti L, Pecorari L, Pucci N, et al. Anaphylaxis to Intravenous Tramadol in a Child. Pharmacology. 2015; 96(5-6), 256–258. https://doi.org/10.1159/000441005
11. Hallberg P, Brenning G. Angioedema induced by trama-dol-a potentially life-threatening condition. European journal of clinical pharmacology, 2005; 60(12): 901–903. https://doi.org/10.1007/s00228-004-0882-5
12. Li PH, Ue KL, Wagner A, Rutkowski R, Rutkowski K. Opioid Hypersensitivity: Predictors of Allergy and Role of Drug Provocation Testing. The journal of allergy and clinical immunology. In practice. 2017; 5(6): 1601–1606. https://doi.org/10.1016/j.jaip.2017.03.035
13. Nasser SM, Ewan PW. Opiate-sensitivity: clinical charac-teristics and the role of skin prick testing. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 2001; 31(7), 1014–1020. https://doi.org/10.1046/j.1365-2222.2001.01090.x
14. Baldo BA. Opioid Analgesic Drugs: Misuse, Toxicity, and Hypersensitivity. The journal of allergy and clinical immunology: In practice. 2017; 5(6): 1607–1608. https://doi.org/10.1016/j.jaip.2017.04.028
15. Sánchez-González MJ, Barbarroja-Escudero J, Antolín-Amérigo D, Rodríguez-Rodríguez M, Pericet L, Medina I, et al. Erythema Multiforme Induced by Tramadol: An Allergy Assessment. Journal of investigational aller-gology & clinical immunology. 2020; 30(4): 290–291. ht-tps://doi.org/10.18176/jiaci.0495
16. Sahutoglu C, Kocabas S, Askar FZ. Tramadol use in a patient with Brugada syndrome and morphine allergy: a case report. Journal of pain research. 2018; 11: 191-194. https://doi.org/10.2147/JPR.S150905
17. Wahler RG, Smith DB, Mulcahy KB. Nebulized Fentanyl for Dyspnea in a Hospice Patient with True Allergy to Morphine and Hydromorphone. Journal of pain & palliative care pharmacotherapy. 2017; 31(1): 38-42. https://doi.org/10.1080/15360288.2017.1279499
18. Kalangara J, Potru S, Kuruvilla M. Clinical Manifestations and Diagnostic Evaluation of Opioid Allergy Labels - A Review. Journal of pain & palliative care pharmacothera-py. 2019; 33(3-4): 131–140. https://doi.org/10.1080/15360288.2019.1666955
19. Li PH, Ue KL, Wagner A, Rutkowski R, Rutkowski K. Opioid Hypersensitivity: Predictors of Allergy and Role of Drug Provocation Testing. The journal of allergy and clinical immunology: In practice. 2017; 5(6), 1601-1606. https://doi.org/10.1016/j.jaip.2017.03.035
20. Sommerfield DL, Sommerfield A, Schilling A, Slevin L, Lucas M, Von Ungern-Sternberg BS. Allergy alerts - The incidence of parentally reported allergies in children presenting for general anesthesia. Paediatric anaesthesia. 2019; 29(2): 153-160. https://doi.org/10.1111/pan.13541
21. Kalangara J, Potru S, Kuruvilla M. Clinical Manifestations and Diagnostic Evaluation of Opioid Allergy Labels - A Review. Journal of pain & palliative care pharmacotherapy. 2019; 33(3-4): 131–140. https://doi.org/10.1080/15360288.2019.1666955
22. Powell MZ, Mueller SW, Reynolds PM. Assessment of Opioid Cross-reactivity and Provider Perceptions in Hospitalized Patients With Reported Opioid Allergies. The Annals of pharmacotherapy. 2019; 53(11): 1117-1123. https://doi.org/10.1177/1060028019860521
23. Benkhaial A, Kaltschmidt J, Weisshaar E, Diepgen TL, Haefeli WE. Prescribing errors in patients with documented drug allergies: comparison of ICD-10 coding and written patient notes. Pharmacy world & science: PWS. 2009; 31(4): 464-472. https://doi.org/10.1007/s11096-009-9300-5
24. Cabrera-Freitag P, Gastaminza G, Goikoetxea MJ, Lafuente A, De la Borbolla JM, Sanz ML. Immediate allergic reaction to atropine in ophthalmic solution confirmed by basophil activation test. Allergy. 2009; 64(9): 1388-1389. https://doi.org/10.1111/j.1398-9995.2009.02052.x
25. Brockow K, Romano A. Skin tests in the diagnosis of drug hypersensitivity reactions. Current pharmaceutical design. 2008; 14(27): 2778-2791. https://doi.org/10.2174/138161208786369821
26. Dewachter P, Lefebvre D, Kalaboka S, Bloch-Morot E. An anaphylactic reaction to transdermal delivered fentanyl. Acta anaesthesiologica Scandinavica. 2009; 53(8): 1092–1093. https://doi.org/10.1111/j.1399-6576.2009.02022.x
27. Baldo BA, Harle DG. Drug allergenic determinants. Monographs in allergy. 1990; 28: 11-51.
28. López-Marina V, Pizarro-Romero G, Costa-Bardají N. Neumonitis por hipersensibilidad asociada a tramadol [Hypersensitivity pneumonitis associated to tramadol]. Medicina clinica. 2006; 126(2): 77–78. https://doi.org/10.1157/13083574
29. Casale TB, Bowman S, Kaliner M. Induction of human cutaneous mast cell degranulation by opiates and endogenous opioid peptides: evidence for opiate and nonopiate receptor participation. The Journal of allergy and clinical immunology. 1984; 73(6), 775-781. https://doi.org/10.1016/0091-6749(84)90447-0
Descargas
Publicado
Número
Sección
Licencia
Derechos de autor 2020 Adrián Díaz Medina

Esta obra está bajo una licencia internacional Creative Commons Atribución 4.0.