Diffuse large B-cell lymphoma, NOS. Clinicopathological study in a cohort of peruvian patients
DOI:
https://doi.org/10.37711/rpcs.2020.2.3.191Keywords:
Herpesvirus 4 Human, Germinal Center, Peru, Epstein-Barr Virus Infec-tions, Cohort Studies, Lymphoma, B-Cell, Stomach, Biomarkers, Proto-Oncogene Proteins c-BCL-2Abstract
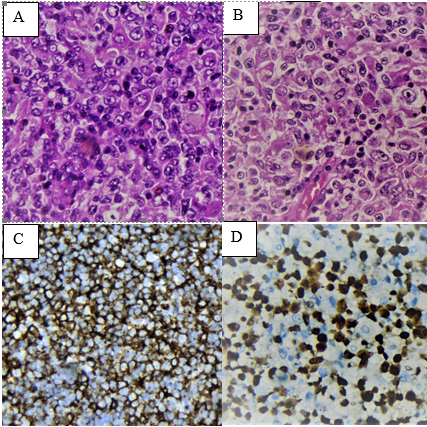
Objective. To evaluate the clinicopathological characteristics of diffuse large B-cell lymphoma, not otherwise specified (LCBGD, NOS), in a cohort of peruvian patients. Methods. Seventy-two cases with a diagnosis of LCBGD, NOS, were studied at the INEN. Clinicopathological characteristics, overall survival (OS) and other clinical parameters were evaluated according to origin cell, Epstein-Barr virus infection (EBER), and the immunohistochemical expression (IHC) of MYC and BCL2. Results. There were 54% women and 46% men, with an average age of 65 years. 76% were ganglionic, the majority cervical location and 24% extranodal, the stomach being the most affected. The most frequent stages were II (35%) and III (29%), the majority with medium-high and high IPI. Histologically, almost all cases had centroblastic and immunoblastic morphology. There was a similar proportion of cases with germinal center (GCB) and non-germinal center (non-GCB) subtypes. GCB and EBER+ had better OS than non-GCB and EBER-, respectively. Likewise, cases with negative IHC for MYC and BCL2 had better OS and a higher percentage in early stages. These findings were not statistically significant. Conclusion.Our cases show characteristics similar to those described in the literature, although with a higher percentage associated with EBV and non-GCB cases. The small number of cases evaluated with IHC to determine subtypes and biomarkers may have limited the statistical analysis in this cohort of cases.
Downloads
References
1. Chan ACL, Chan JKC. Diffuse large B-cell lymphoma. En: Jaffe ES, Arber DA, Campo E, Harris NL, Quintanilla-Martínez L. Hematopathology. 2nd ed. Philadelfia, PA. Saunders/ Elsevier 2016: 415-444.
2. Gascoyne RD, Campo E, Jaffe ES, Chan WC, Chan JKC, Rosendwald A, et al. Diffuse large B-cell lymhoma, NOS. En: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri HS, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, Siebert R. WHO classification of tumors of haematopoie-tic and lymphoid tissues. Revised 4th ed. IARC, Lyon 2017: 291-297.
3. Ministerio de Salud (Perú). Instituto Nacional de Enfer-medades Neoplásicas. Registro de cáncer de Lima Metro-politana 2010-2012. Incidencia y mortalidad, volumen 5. Lima, 2016. [Internet] [Consultado 2020 Ago 26] Dispo-nible en: http://bvs.minsa.gob.pe/local/MINSA/3774.pdf
4. Ferlay J, Ervik M, Lam F, Colombet M, Mery L, Piñeros M, Znaor A, Soerjomataram I, Bray F. Global cancer observa-tory: Cancer today. [Internet] [Consultado 2020 Ago 26] Disponible en : https://gco.iarc.fr/today,accessed
5. López A, Colomo L, Jiménez M, Bosch F, Campo E, Montserrat E, et al. Diffuse large B-cell lymphoma: clinical and biological characterization and outcome according to the nodal or extranodal primary origin.J Clin Oncol, 2005; 23(12): 2797-804.
6. Sukswai N, Lyapichev K, Khoury JD, Medeiros LJ. Diffuse large B-cell lymhoma variants: an update. Pathology. 2020; 52(1): 53-67.
7. Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of dif-fuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000 Feb; 403(6769):503-511.
8. Chang C, MacClintock S, Cleveland RP, Trzpuc T, Vesole DH, Logan B, et al. Immunohistochemical expression pa-tterns of germinal center and activation B-cell markers correlate with prognosis in diffuse large B- cell lympho-ma. Am I Surg Pathol. 2004 Abr; 28 (4).
9. Gascoyne RD. Emerging prognostic factors in diffuse large B cell lymphoma. Curr Opin Oncol. 2004; 16(5): 436-441.
10. Lossos IS, Jones CD, Warnke R, Natkunam Y, Kaizer H, Ze-hnder JL, et al. Expression of a single gene, BCL-6, stron-gly predicts survival in patients with diffuse large B-cell lymphoma. Blood. 2001; 98(4): 945-951.
11. Nakamura S, Jaffe E, Swerdlow SH. EBV positive diffuse large B-cell lymhoma, not otherwise specified (NOS). En: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri HS, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, Siebert R. WHO classification of tumors of haemaopoie-tic and lymphoid tissues. Revised 4th ed. IARC, Lyon 2017: 304-306.
12. Lui Y, Bar SK. Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol. 2019 May; 94(5): 604–616.
13. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri HS, Thiele J, Arber DA, Hasserjian RP, Le Beau MM, Orazi A, Siebert R. WHO classification of Tumors of Haemaopoietic and Lymphoid Tissues. Revised 4th ed., Lyon: International Agency for Research on Cancer; 2017.
14. Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Delabie J, Ott G, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immu-nohistochemistry using a tissue microarray. Blood 2004 Ene; 103: 275–82.
15. Choi WW, Weisenburger DD, Greiner TC, Piris MA, Ban-ham AH, Delabie J, et al. A new immunostain algorithm classifies diffuse large B-cell lymphoma into molecular subtypes with high accuracy. Clin Cancer Res. 2009 Sep 1; 15(17): 5494-502.
16. Lossos IS, Morgensztern D. Prognostic biomarkers in diffuse large B-cell lymphoma. J Clin Oncol. 2006; 24(6): 995-1007.
17. Rosenwald A, Wright G, Chan WC, Connors JM, Campo E, Fisher RI, et al. Lymphoma/leukemia molecular profiling project. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346(25): 1937-947.
18. Bea S, Zettl A, Wright G, Salaverria I, Jehn P, Moreno V, et al. Lymphoma/leukemia molecular profiling project. Diffuse large B-cell lymphoma subgroups have distinct genetic profiles that influence tumor biology and improve gene-expression-based survival prediction. Blood. 2005;106(9): 3183-190.
19. Zhang A, Ohshima K, Sato K, Kanda M, Suzumiya J, Shi-mazaki K, et al. Prognostic clinicopathologic factors, including immunologic expression in diffuse large B-cell lymphomas. Pathol Int. 1999; 49(12): 1043-52.
20. Yan LX, Liu YH, Luo DL, Zhang F, Cheng Y, Luo XL, et al. MYC expression in concert with BCL2 and BCL6 expres-sion predicts outcome in chinese patients with diffuse large B-cell lymphoma, not otherwise specified PLoS One. 2014; 9(8): e104068. doi: 10.1371/journal.pone.0104068.
21. Castillo JJ, Beltran BE, Miranda RN, Young KH, Chavez JC, Sotomayor EM. EBV-positive diffuse large B-cell lymphoma of the elderly: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol. 2016; 91(5): 529-37. doi: 10.1002/ajh.24370.
22. Harada S, Suzuki R, Uehira K, Yatabe Y, Kagami Y, Ogura M, et al. Molecular and immunological dissection of diffuse large B cell lymphoma: CD5+, and CD5- with CD10+ groups may constitute clinically relevant subtypes. Leuke-mia. 1999 Sep; 13(9): 1441-7. doi: 10.1038/sj.leu.2401487.
23. Barrans SL, O’Connor SJ, Evans PA, Davies FE, Owen RG, Haynes AP, et l.: Rearrangement of the BCL6 locus at 3q27 is an independent poor prognostic factor in nodal diffuse large B-cell lymphoma. Br J Haematol. 2002 May; 117(2): 322-32. doi: 10.1046/j.1365-2141.2002.03435.x.
24. Ree HJ, Yang WI, Kim CW, Huh J, Lee SS, Cho EY, et al. Coexpression of BCL-6 and CD10 in diffuse large B-cell lymphomas: significance of BCL-6 expression patterns in identifying germinal center B-cell lymphoma. Hum Pathol. 2001 Sep; 32(9): 954-62. doi: 10.1053/hupa.2001.27118.
25. Akyurek N, Uner A, Benekli M, Barista I, et al. Prognos-tic significance of MYC, BCL2, and BCL6 rearrangements in patients with diffuse large B-cell lymphoma treated with cyclophosphamide, doxorubicin, vincristine, and prednisone plus rituximab. Cancer. 2012 Sep 1; 118(17): 4173-83. doi: 10.1002/cncr.27396.
26. Curry CV, Ewton AA, Olsen RJ, Logan BR, Preti HA, Liu YC, et al. Prognostic impact of C-REL expression in diffuse large B-cell lymphoma. J Hematop. 2009; 2(1): 20-26. doi: 10.1007/s12308-009-0021-4.
27. Guo Y, Takeuchi I, Karnan S, Miyata T, Ohshima K, Seto M. Array-comparative genomic hybridization profiling of immunohistochemical subgroups of diffuse large B-ce-lllymphoma shows distinct genomic alterations. Cancer Sci. 2014 Apr; 105(4): 481-9. doi: 10.1111/cas.12378.
28. Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and pathogenesis of diffu-se large B-cell lymphoma. N Engl J Med. 2018 Apr 12; 378(15): 1396-1407. doi: 10.1056/NEJMoa1801445.
29. Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic me-chanism and outcomes. Nat Med. 2018 May; 24(5): 679-690. doi: 10.1038/s41591-018-0016-8.
30. Beltran BE, Castro D, Paredes S, Miranda RN, Castillo JJ. EBV-positive diffuse large B-cell lymphoma not otherwi-se specified: 2020 update on diagnosis, risk-stratifica-tion, and management. Am J Hematol. 2020 Abr; 95(4): 435–445. https://doi.org/10.1002/ajh.25760.
31. Shimoyama Y, Yamamoto K, Asano N, Oyama T, Kinoshi-ta T, Nakamura S. Age-related Epstein-Barr virus-associa-ted B-cell lymphoproliferative disorders: special referen-ces to lymphomas surrounding this newly recognized clinicopathologic disease. Cancer Sci. 2008 Jun; 99(6): 1085-91. doi: 10.1111/j.1349-7006.2008.00813.x.
32. Oyama T, Yamamoto K, Asano N, Oshiro A, Suzuki R, Ka-gami Y, et al. Age-Related EBV-Associated B-Cell Lym-phoproliferative Disorders Constitute Distinct Clinicopathologic Group:A Study of 96 Patients. Clin Cancer Res. 2007 Sep 1; 13(17): 5124-32. doi: 10.1158/1078-0432.CCR-06-2823
33. Park S, Lee J, Ko YH, Han A, Jun HJ, Lee SC, et al. The im-pact of Epstein Barr Virus on clinical outcome in diffuse large B cell lymphoma. Blood. 2007 Aug 1; 110(3): 972-8. doi: 10.1182/blood-2007-01-067769
34. Xu FP, Liu Y, Zhuang H, Luo D, Li L, Zhang F, et al. Clinicopathological features of Epstein-Barr virus-positive diffuse large B-cell lymphoma in elderly. Zhonghua Bing Li Xue Za Zhi. 2011 Sep; 40(9): 616-21.
35. Ting CY, Chang KM, Kuan JW, Sathar J, Chew LP, Wong OLJ, et al. Clinical Significance of BCL2, C-MYC, and BCL6 genetic abnormalities, Epstein-Barr Virus Infection, CD5 protein expression, germinal center B cell/non-germi-nal center B-cell subtypes, co-expression of MYC/BCL2 proteins and co-expression of MYC/BCL2/BCL6 proteins in diffuse large B-cell lymphoma: A clinical and patho-logical correlation study of 120 patients. Int. J. Med. Sci. 2019; 16(4): 556-566.
Downloads
Published
Issue
Section
License
Copyright (c) 2020 Daniela Dueñas Hancco, Patricia Arboleda Ezcurra, Sandro Casavilca-Zambrano, Daniel Enríquez Vera, Raúl Mantilla Quispe, Carlos Barrionuevo Cornejo

This work is licensed under a Creative Commons Attribution 4.0 International License.